First Cryo-electron microscopy suite in the ACT
ANU’s Centre for Advanced Microscopy (CAM) has unveiled its $7.5million suite of cryo-electron microscopes—the first of its kind in the ACT—which will support research ranging from disease control and food security to recycling and clean energy technology development.
Cryo-electron microscopy (cryo-EM) has revolutionised life sciences, as it allows cells and biological molecules to be observed in their near-live state (i.e. without chemical fixation and staining). The researchers who discovered the technique were awarded the Nobel Prize for Chemistry in 2017, and since then cryo-EM has become increasingly important for atomic-scale structural analysis of individual molecules such as proteins, complexes and virus particles. It has, for example, been a crucial tool in identifying the structure of the SARS-CoV2 virus (e.g. Subramaniam, IUCrJ. 2020). Cryo EM is also used for structural analysis of temperature-, energy- or environment-sensitive materials.
Researchers are now able to access the full suite of cryo instruments at CAM, which includes a Focused Ion Beam/Scanning Electron Microscope (ZEISS Crossbeam 550 FIB-SEM), a screening Transmission Electron Microscope (the JEOL JEM F200) and a dedicated cryo-TEM with an autoloader (the JEOL Cryo ARM™ 200) (Figure 1). Co-funded by Microscopy Australia (via the National Collaborative Research Infrastructure Strategy (NCRIS)) and the ANU, the new instruments are multi-functional, highly automated and can operate independently or as part of a correlated workflow. CAM also houses all the necessary equipment for cryo sample preparation, including the Leica EM GP plunger for cryo-freezing of grids, as well as a fast and sophisticated processing pipeline.
The new Cryo ARM™ 200 with its ability to store frozen grids, and exchange up to 12 specimen samples at a time, specialises in efficient, automated biological imaging and processing. The instrument features a cold field emission gun and in-column energy filter, both of which minimise background ‘noise’ to produce a clearer image of molecules such as proteins and molecular complexes. It also includes automated software for acquisition of single particle analysis (SPA) data. Thousands of 4k x 4k digital images can be collected using a Direct Electron detection (DE64) camera. Post processing can be performed on either an “in house” 8 GPU cluster, or through the National Computational Infrastructure (NCI Australia) facilities. Since installation of the Cryo ARM™ 200, CAM staff have imaged and resolved the structure of the mouse Apoferritin complex down to 2.8 Å resolution (Figure 2). This instrument can also view cryo lamella samples prepared using the ZEISS Crossbeam 550 FIB-SEM.
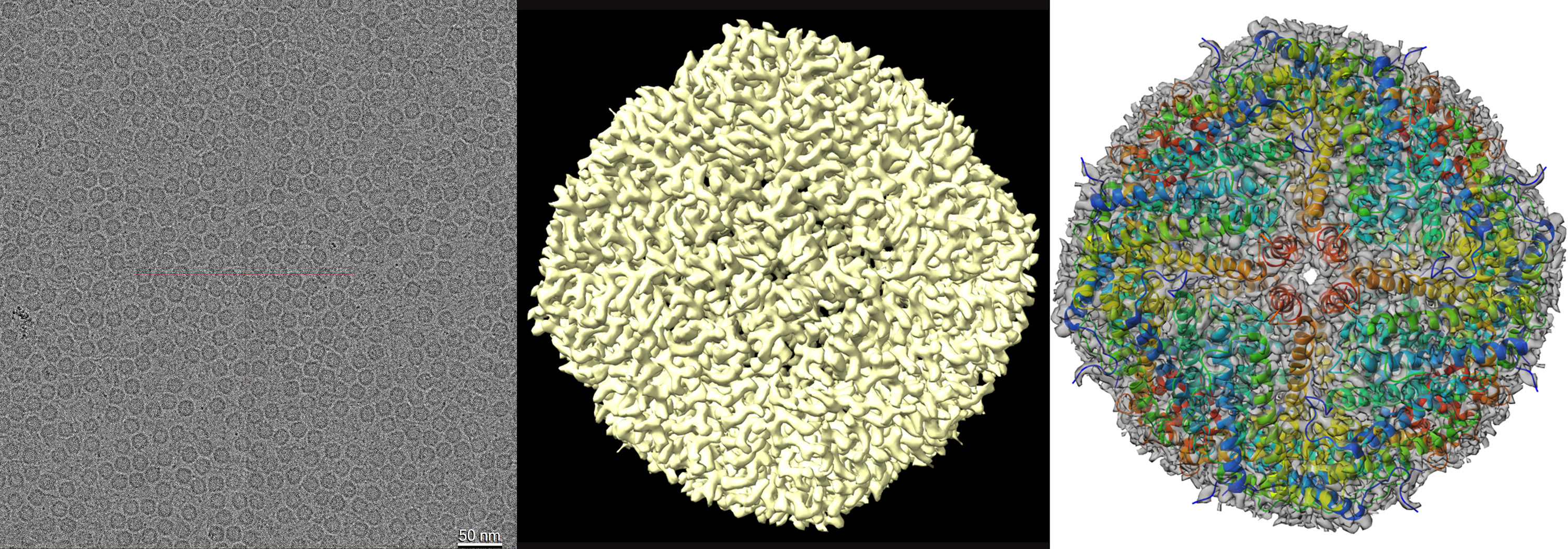
Figure 2: Cryo-EM structure of mouse apoferritin. (a) Image of apoferritin captured using Cryo-ARM 200 fitted with a DE64 camera (b). EM density map of apoferritin complex at 2.86 Å resolution. (c) The refined atomic coordinates of the apoferritin complex (cartoon representation) fitted into the cryo-EM density map at 50% transparency.
Using its focused ion beam, the ZEISS Crossbeam 550 FIB-SEM can generate thin, structurally intact samples (lamella) from cells and tissues, suitable for TEM examination (tomography studies both at RT and under cryo conditions). Furthermore, the Crossbeam can perform block face imaging, a technique whereby a room temperature or frozen sample has thin layers progressively shaved off. Images of the sample (block-face) are captured after each layer is removed, which can then be compiled to form a 3D rendering of the volume of the sample (see examples of cryo volume imaging and RT volume imaging). The range of capabilities available on the Crossbeam combined with the expertise of CAM staff renders this instrument a valuable resource for both physical and life sciences research.
To evaluate the conditions of samples (e.g. concentration, homogeneity) for SPA analysis, the JEOL JEM-F200 TEM can be used as a screening tool at either room temperature, or under cryo conditions, prior to studying samples on the Cryo ARM 200. In addition, the JEOL JEM-F200 can cater for a broad range of other research requirements. This TEM can be operated at 80, 120 and 200 kV to provide optimal conditions for a large variety of samples from both the materials and life sciences. It features an intuitive user interface as well as an easy to use, semi-automated, sample holder loading and unloading device. Like the Cryo ARM™ 200, the F200 has a cold field emission gun providing high beam stability and optimum spatial resolution. It is equipped with a Gatan Rio16 camera for routine imaging (and Micro Electron Diffraction) as well as a Direct Electron detection (DE16) camera for low dose imaging and SPA pre-screening.
CAM has also upgraded one of its confocal microscopes (ZEISS LSM800 /Airyscan) with a Linkam Scientific cryo stage (CMS196) and ZEISS Atlas 5 and ZEN Connect software to provide a multimodal correlative light and electron microscopy (CLEM) workflow (Figure 3). This multimodal pipeline enables users to view their samples across multiple scales and imaging platforms by combining the advantages of light microscopy (e.g. speed of analysis, flexibility and molecular specificity through fluorescence imaging) with the high-resolution capability of electron microscopy. The highly sophisticated ZEN Connect software allows a semi-automated correlative workflow, combining images from both modalities (CLEM) by overlaying fluorescence or transmission imaging information with ultrastructural precision.

Figure 3: The multimodal correlative light and electron microscopy (CLEM) workflow at the Centre for Advanced Microscopy, ANU.
If you are interested in accessing any of CAM’s instruments and/or expertise to support your research please contact Melanie Rug (melanie.rug@anu.edu.au).